Process
Prepared items are galvanized by immersion in molten zinc. The surface of the work is completely covered, producing a uniform coating of zinc and zinc-iron alloy layers whose thickness is determined principally by the mass of the steel being galvanized. This is an important advantage of the galvanizing process – a standard minimum coating thickness is applied automatically.

The molten zinc in the galvanizing bath covers corners, seals edges, seams and rivets, and penetrates recesses to give complete protection to areas which are potential corrosion spots with other coating systems. The galvanized coating is slightly thicker at corners and narrow edges, giving greatly increased protection compared to organic coatings which thin out in these critical areas.
All material processed by Galco Steel is in accordance with I.S. EN ISO 1461:2022
Preparation
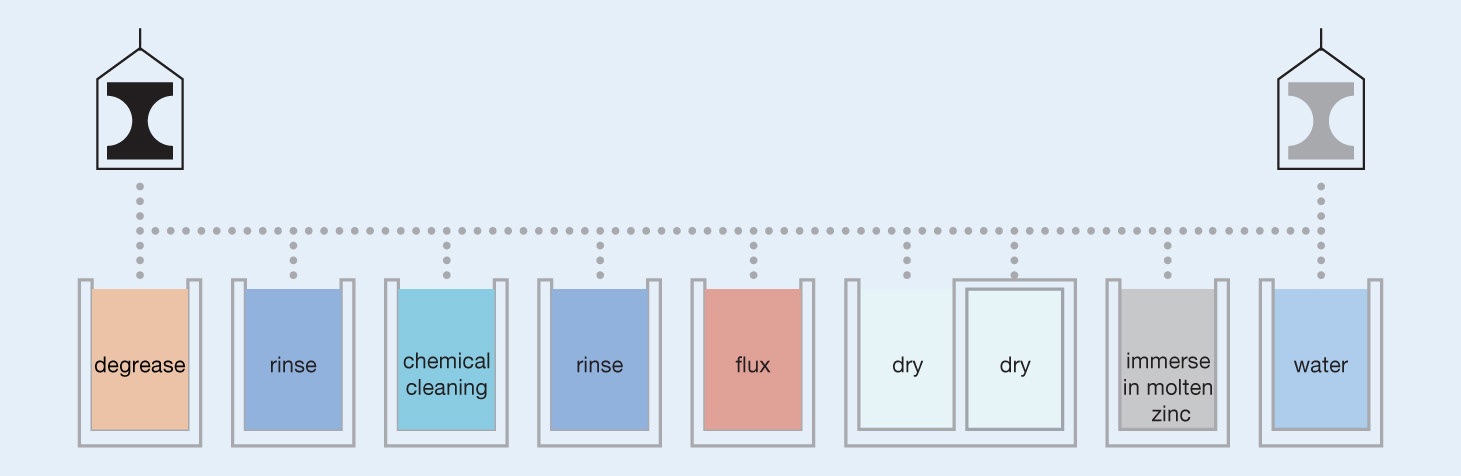
The galvanizing reaction will only occur on a chemically clean surface. Like most coating processes the secret to achieving a good quality coating lies in the preparation of the surface. It is essential that this is free of grease, dirt and scale before galvanizing. Visual inspection of galvanized products shows that work is completely protected and gives an excellent guide to overall coating quality.
Contamination is removed by a series of processes. Firstly, we degrease using a caustic solution into which the component is dipped. The article is then rinsed and then dipped in hydrochloric acid at ambient temperature to remove rust and mill scale. Welding slag, paint and heavy grease will not be removed by these cleaning steps and should be removed before the work is sent to the galvanizer.
After further rinsing, the components are then dipped in a flux solution – usually about 30% zinc ammonium chloride at around 65-80°C.
Galvanizing
When the clean iron or steel component is dipped into the molten zinc (at 450°C) a series of zinc-iron alloy layers are formed by a metallurgical reaction between the iron and zinc. Upon withdrawal from the galvanizing bath a layer of molten zinc will be taken out on top of the alloy layer. This cools to exhibit the bright shiny appearance associated with galvanized products.
Quench
Items are quenched in a mild sodium dichromate solution to prevent the onset of wet storage staining during the early life of galvanizing.
Final inspection
The newly galvanized steel is sight-inspected and hand filed to the end use requirements.
Coating
When the reaction between iron and zinc has ceased and the article is taken out of the galvanizing bath complete with its outer coating of zinc, the process is complete. In reality there is no demarcation between steel and zinc but a gradual transition through the series of alloy layers which provide the metallurgical bond.
Performance
Toughness – the coating bonds metallurgically with the steel giving a much greater resistance to damage than other coatings.
Complete coverage – because it is dipped in molten zinc, all parts of the surfaces are coated – inside and out – including awkward corners and narrow gaps.
Three Way Protection – Hot Dip Galvanizing protects in three ways.
It weathers at a slow rate giving a long and predictable life. The coating sacrifices itself to any small areas exposed through drilling, cutting or accidental damage. If large areas get damaged, it prevents the sideways creep of rust.
Contact our team
Over the past 50 years, Galco has become a recognized leader offering high quality equipment and systems. We are dedicated to exploring the latest construction technologies to generate cost savings for clients.
Send Us a Message
Fields marked with an * are required
"*" indicates required fields



